Strategic Shifts Redefining First-In-Human Study Frameworks
Across the global life sciences industry, the early stages of clinical development are increasingly recognized as decisive inflection points that determine the long-term trajectory of therapeutic candidates. Organizations entering this first-in-human phase must navigate a complex landscape where safety, dose escalation, and biological response patterns are carefully measured. As therapies targeting previously inaccessible pathways emerge, sponsors not only require specialized study environments but also depend on teams experienced in managing challenging designs with precision. This shift reflects the rising urgency to accelerate innovation without compromising ethical standards or data reliability.
The expanding role of the First In Human Services Market demonstrates how vital specialized partners have become in managing the scientific and operational intricacies of first-dose research. Sponsors rely on these services for comprehensive safety monitoring, pharmacokinetic evaluations, real-time analytics, and adaptive protocol management. As therapies become more individualized, early-phase providers assist researchers in designing dosing regimens that reflect mechanistic insights gathered from preclinical modeling. These capabilities reduce risk, support regulatory readiness, and empower development teams with greater confidence when advancing into more extensive clinical phases.
Competitive dynamics across the sector are increasingly shaped by evolving First In Human Services Market Share trends. These patterns reveal which regions and service providers are gaining prominence as trusted early-phase collaborators. Research organizations with strong regulatory track records, modern clinical units, and multidisciplinary expertise continue to capture a growing portion of sponsor demand. Global partnerships and expansions into emerging trial regions further strengthen competitive positioning by offering sponsors greater geographic flexibility and improved patient-recruitment pathways.
Technology continues to redefine how early-phase studies are executed, allowing researchers to leverage advanced digital frameworks for monitoring, data interpretation, and predictive modeling. Artificial intelligence enables rapid identification of safety trends, while remote-monitoring platforms ensure continuous oversight without interrupting study flow. These tools enhance operational efficiency and improve decision-making accuracy, especially in studies with complex pharmacological endpoints.
Ethical and regulatory expectations also remain central, particularly given the sensitive nature of first-in-human exposure. Leading providers adopt stringent safety measures that exceed minimum compliance standards, reinforcing participant protection and ensuring transparent data reporting. This emphasis on accountability strengthens trust among regulatory agencies, trial participants, and sponsors.
The growing demand for early-phase excellence is set to intensify in the coming years as breakthrough therapies in oncology, rare diseases, neurology, and immunology progress toward initial human testing. With the global research environment becoming increasingly interconnected, sponsors seek early-phase partners capable of blending innovation with operational discipline. The continued evolution of first-in-human services will play an essential role in shaping how rapidly and safely new therapies advance toward broader clinical evaluation.
Related Reports:
Immersion Long Working Distance Objective Market
Magnetic Rotary Encoder Modules Market
Antimicrobial Low Voc Latex Paint Market
Pediatric Electrolyte Supplements Injection Market
Autonomous Delivery Network Market
Antimicrobial Zero Voc Latex Paint Market
Teen Gymnastics Leotards Market
For more in-depth research insights, visit Infinity Market Research.
Stay informed with the latest updates on News Innings, Researcher Diaries, and Industry News Desk.
Categories
Read More
Participating in the Last War: Survival event offers players a strategic opportunity to earn valuable courage medals, a special currency that enhances gameplay progression. These medals are primarily obtained by engaging in the biweekly invading zombie challenge, which pits players against waves of undead enemies and formidable zombie bosses. During the event, invading zombies spawn randomly...

The chemical sector remains resurgent, delivering critical inputs in agriculture, healthcare, construction, and consumer uses. With increasing demand for specialty solutions and green products, the sector moves forward incrementally. Growth between 2025 to 2031 will be at a CAGR rate of 4.2% and is strongly connected to the industries in need of safe and secure material innovation. At the core...

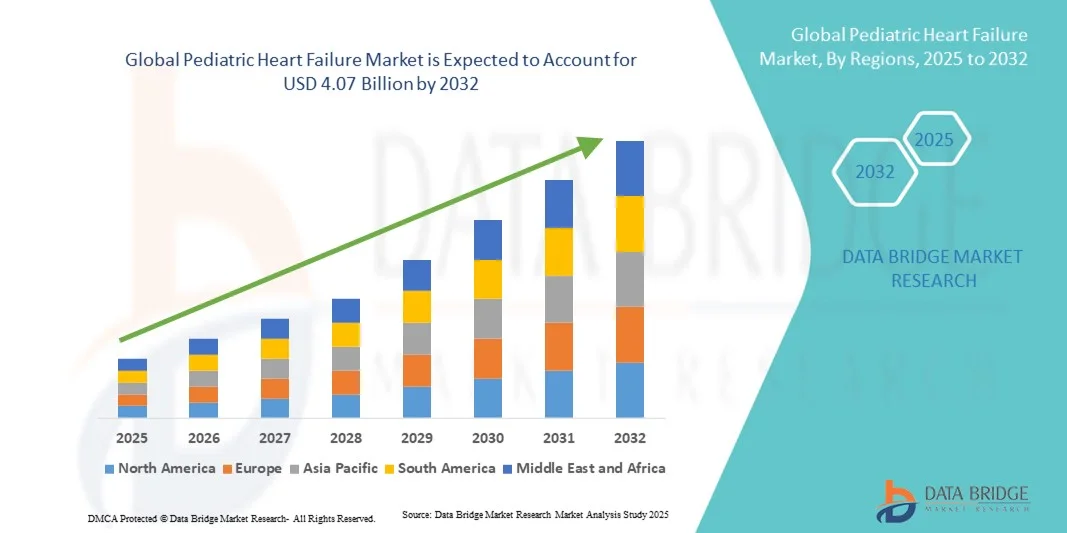
"What’s Fueling Executive Summary Pediatric Heart Failure Market Size and Share Growth CAGR Value The global pediatric heart failure market size was valued at USD 2.78 billion in 2024 and is expected to reach USD 4.07 billion by 2032, at a CAGR of 4.90% during the forecast period The market growth is largely fueled by the increasing prevalence of...
The competitive arena blends incumbent telco software vendors, cloud platform providers, and automation specialists. According to Service Fulfilment Competitive Landscape, differentiation hinges on catalog governance, intent-based orchestration, model-driven inventory, and Open API ecosystems. Vendors with proven public cloud scale, robust DevOps pipelines, and prebuilt process...
The automobile sector is still one of the most crucial sectors shaping industrial as well as consumer economies globally. Innovation, manufacturing size, and demand flexibility continue to keep this sector in the limelight regardless of temporary setbacks. From 2025 to 2031, model estimates suggest that the sector will expand at a CAGR rate of 29.5% from 2023 to 2030 with valuation trends...



