Strategic Shifts Redefining First-In-Human Study Frameworks
Across the global life sciences industry, the early stages of clinical development are increasingly recognized as decisive inflection points that determine the long-term trajectory of therapeutic candidates. Organizations entering this first-in-human phase must navigate a complex landscape where safety, dose escalation, and biological response patterns are carefully measured. As therapies targeting previously inaccessible pathways emerge, sponsors not only require specialized study environments but also depend on teams experienced in managing challenging designs with precision. This shift reflects the rising urgency to accelerate innovation without compromising ethical standards or data reliability.
The expanding role of the First In Human Services Market demonstrates how vital specialized partners have become in managing the scientific and operational intricacies of first-dose research. Sponsors rely on these services for comprehensive safety monitoring, pharmacokinetic evaluations, real-time analytics, and adaptive protocol management. As therapies become more individualized, early-phase providers assist researchers in designing dosing regimens that reflect mechanistic insights gathered from preclinical modeling. These capabilities reduce risk, support regulatory readiness, and empower development teams with greater confidence when advancing into more extensive clinical phases.
Competitive dynamics across the sector are increasingly shaped by evolving First In Human Services Market Share trends. These patterns reveal which regions and service providers are gaining prominence as trusted early-phase collaborators. Research organizations with strong regulatory track records, modern clinical units, and multidisciplinary expertise continue to capture a growing portion of sponsor demand. Global partnerships and expansions into emerging trial regions further strengthen competitive positioning by offering sponsors greater geographic flexibility and improved patient-recruitment pathways.
Technology continues to redefine how early-phase studies are executed, allowing researchers to leverage advanced digital frameworks for monitoring, data interpretation, and predictive modeling. Artificial intelligence enables rapid identification of safety trends, while remote-monitoring platforms ensure continuous oversight without interrupting study flow. These tools enhance operational efficiency and improve decision-making accuracy, especially in studies with complex pharmacological endpoints.
Ethical and regulatory expectations also remain central, particularly given the sensitive nature of first-in-human exposure. Leading providers adopt stringent safety measures that exceed minimum compliance standards, reinforcing participant protection and ensuring transparent data reporting. This emphasis on accountability strengthens trust among regulatory agencies, trial participants, and sponsors.
The growing demand for early-phase excellence is set to intensify in the coming years as breakthrough therapies in oncology, rare diseases, neurology, and immunology progress toward initial human testing. With the global research environment becoming increasingly interconnected, sponsors seek early-phase partners capable of blending innovation with operational discipline. The continued evolution of first-in-human services will play an essential role in shaping how rapidly and safely new therapies advance toward broader clinical evaluation.
Related Reports:
Immersion Long Working Distance Objective Market
Magnetic Rotary Encoder Modules Market
Antimicrobial Low Voc Latex Paint Market
Pediatric Electrolyte Supplements Injection Market
Autonomous Delivery Network Market
Antimicrobial Zero Voc Latex Paint Market
Teen Gymnastics Leotards Market
For more in-depth research insights, visit Infinity Market Research.
Stay informed with the latest updates on News Innings, Researcher Diaries, and Industry News Desk.
Kategorien
Mehr lesen
Executive Summary This report provides a comprehensive overview of the UAE Automotive Aftermarket market, analyzing size, growth trends, key segments, and competitive dynamics. It highlights market drivers, challenges, and emerging opportunities while offering actionable insights for strategic decision-making. Historical data, current analysis, and future forecasts are included to help...

Blizzard has released patch 2.4.0 along with Season 10! Find out all the details about the initial hotfix now live on the servers, only on Maxroll. Be sure to explore the comprehensive Season 10 FAQ, expertly compiled by Wudijo, to stay informed about all the updates and changes. Players may have noticed that legendary items now drop at a more balanced rate across different levels and...

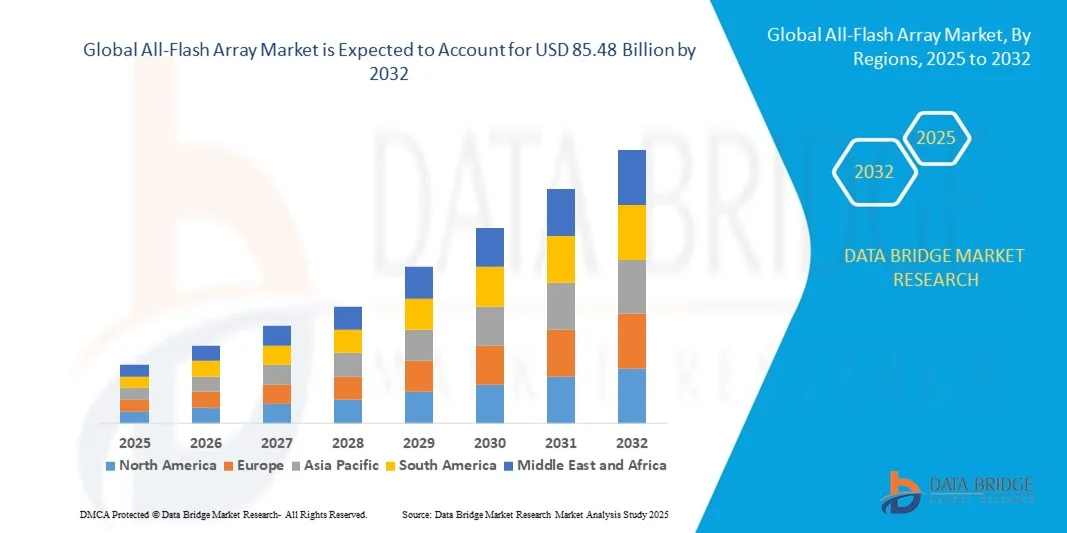
"Latest Insights on Executive Summary All-Flash Array Market Share and Size CAGR Value The global all-flash array market size was valued at USD 19.23 billion in 2024 and is projected to reach USD 85.48 billion by 2032, growing at a CAGR of 20.50% during the forecast period. To produce the best market research report, a wide range of objectives is required to...
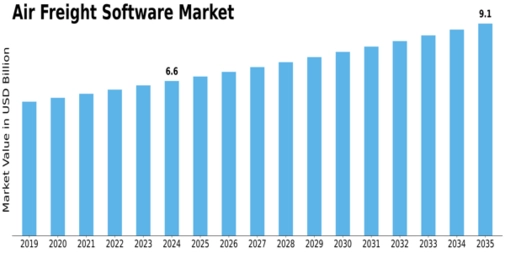
The Air Freight Software Market is experiencing steady and promising growth, underpinned by the exponential rise in global trade and the digital transformation of logistics. According to MRFR, the market was valued at USD 6.63 billion in 2024 and is expected to reach USD 9.09 billion by 2035, with a compound annual growth rate (CAGR) of 2.91% from 2025 to 2035. This growth reflects increasing...
Is your computer potentially compromised by the Conficker worm? A recent advisory from CERT issued over the weekend highlights a simple way to check. If you find yourself unable to access two specific websites—one hosted by Symantec and another by McAfee—it could be a sign that your system is infected, as Conficker is known to block access to these security sites. Windows users are...



